CLOSE
About Elements
Creating technology to support society
for a beautiful future
TANAKA is a precious metals specialist that excels at delivering innovation to the world that brings value to society. “Elements” is an online media circulated by TANAKA Precious Metals that focuses on technology and sustainability information in line with the business and values of the company. It provides hints for creating a better society and prosperous earth for the future in response to the rapid paradigm shift of the modern world.

Description: Recycling Process for Precious Metals

Clean Technology, May 2021
Hirotaka Ikuta, TANAKA Kikinzoku Kogyo K.K.
Introduction
Precious metal is a generic term for gold, silver and platinum-group metals (platinum, palladium, rhodium, iridium, ruthenium and osmium). These metals are characterized by their beautiful metallic luster, long-lasting luster through their chemical stability, and high prices due to small production amounts.1) Owing to these characteristics, they have been used as jewelry and physical assets since ancient times. More recently, however, precious metals are being increasingly used in various industrial fields because their unique physical and chemical properties are valued from industrial viewpoints.
For example, gold is used as material for bonding wires and electric contacts in the electronics industry because of its chemical stability in the atmosphere, excellent malleability and electric conductivity. Moreover, in the medical field, colloidal gold particles (fine gold particles) are used as reagents to be incorporated in extracorporeal diagnostic agents and testing kits.2)
The catalytic action of platinum allows it to be used as a catalyst for automobile exhaust gas purification and electrode catalyst of fuel cells. It is also used for many medical applications: anticancer drugs such as oxaliplatin and cisplatin, pacemaker electrodes, catheter markers, and stopping/prosthetic material for cerebral aneurysms.2)
In many other industrial fields such as chemistry, glass manufacturing and aerospace, and advanced technologies such as hydrogen energy and IoT, precious metals are used intensively for materials development.
Manufacturing and using precious metal products gives rise to process scrap and waste materials produced at the time of manufacturing. These are called “precious metal containing wastes” (hereinafter referred to as “wastes”), and are handled as “urban mines” or “valuables” and assessed, recovered and refined to be recycled into products.
Forms of precious metal containing wastes depend greatly on the materials, structures and manufacturing processes of the products from which the wastes originate. Precious metals are expensive so efforts have been made to use smaller amounts of precious metals or substitute alternative materials, and the concentrations of precious metals in precious metal containing wastes are generally declining year by year. As precious metals are increasingly used for products such as fluorine-containing products and new semiconductor products that have not been handled before, it has become important to develop and apply new recycling technologies adapted to specific wastes, in addition to the use of the existing recycling processes.
This report first gives an overview of the recycling of precious metals and then discusses types of wastes which have increased recently and specific recycling issues for each type of waste.
Overview of precious metals recycling
1. Demand and supply of precious metals
We will take gold as a representative example of precious metals to describe the supply and demand situation.
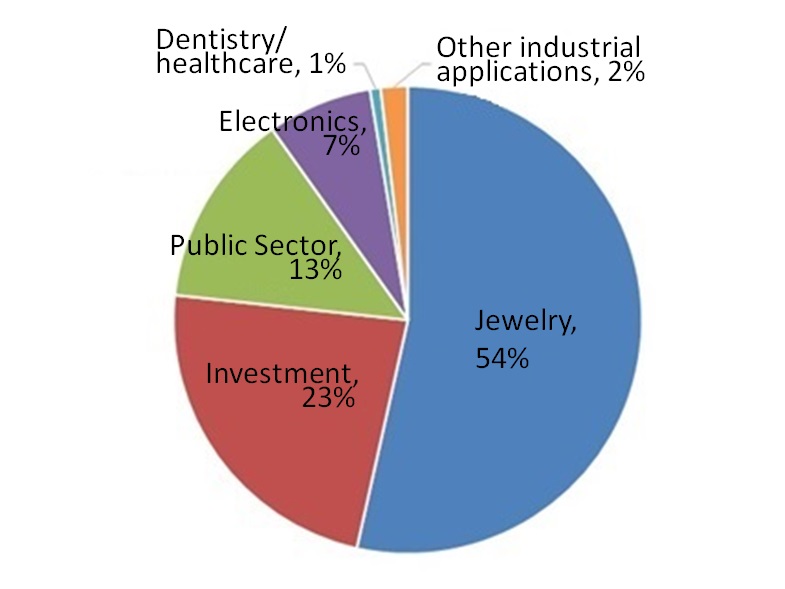
Figure 1 shows the global demand for gold classified by application in 2018. The application to jewelry ranks first. Gold, a symbol of wealth, beauty and power due to its scarcity, continues to be widely handled. Second is the application to investment. In contrast to paper assets such as bank bills or bonds, gold has universal value and involves a lower credit risk (risk of confidence being lost in the issuing entity so that issued bills and bonds become of no value) and is held for long-term conservation of assets. 4)
Figure 2 shows the domestic demands for gold classified by application in Japan in 2019. In Japan, demand for gold is more intensive for applications as an industrial material in industries such as electronics and plating than for jewelry and investment. This is because Japan is a country which produces electrical equipment and precision machinery, and gold is used in such products.
| Figure 1: Demand for Gold by Application (total: 3,979 tons in 2018, global demand3) ) |
Figure 2: Demand for Gold by Application (total: 84 tons in 2019, domestic demand in Japan5) ) |
|---|---|
 |
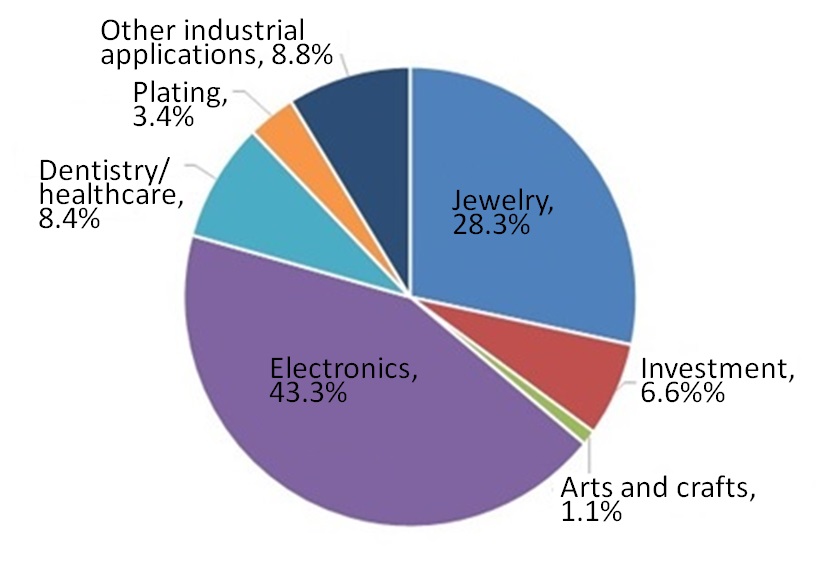 |
Figure 3 shows the trends in Japan of the demand for gold classified by application for the eight-year period 2012 to 2019. Despite a considerable decrease for several years after 2012, the demand for gold gradually recovered to 84 tons in 2019, 91% of the amount of demand in 2012. The demand for gold for industrial applications has tended to increase in recent years but the amount of gold used per product is decreasing. 6) This is probably because of reduction in the utilization of gold and shifting to alternative materials for the purpose of reducing manufacturing costs.
Figure 3: Trends of Demand for Gold by Application (2012-2019, Japan)5)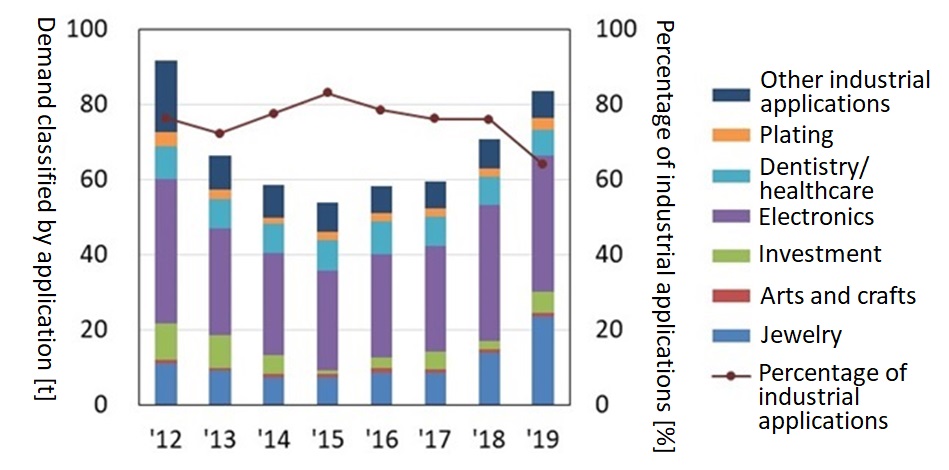 * Percentage of industrial applications: value as result obtained by dividing the demand for gold for electronics, dentistry/healthcare and plating by demand for gold. |
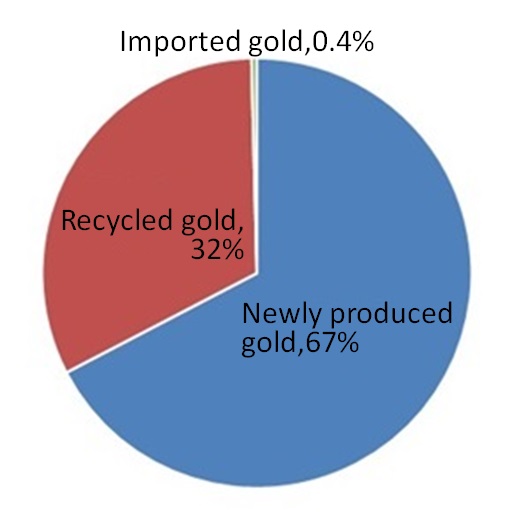
Figure 4 shows the percentages of different supply sources of gold in Japan in 2019. Remanufactured gold makes up 32 percent of the annual supply (162 tons) and newly-produced gold accounts for the majority of the supply.
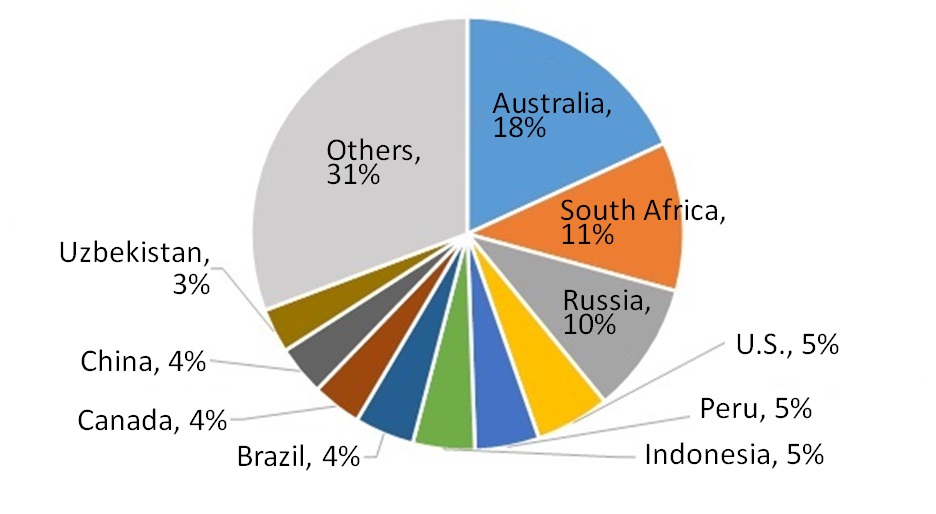
Figure 5 shows percentages of gold reserves in major countries. Australia is ranked first, followed by South Africa, Russia, the U.S. and so on. These top four countries account for 44 percent of the global reserves.
Japan produces 7 tons of gold annually. A majority of the 7 tons is produced at the Hishikari Mine in Kagoshima Prefecture. 8) The estimated reserves of gold in that mine are 250 tons. 9) This means that newly produced domestic gold alone is not able to meet the entire domestic demand. Domestic industries cannot do without imported gold. Nonetheless, it should be noted that a considerable amount of gold has been accumulated in urban mines. An amount of 6,800 tons of gold is estimated to exist in urban mines. 10) It corresponds to 80 years of domestic demand for gold. Thus, recycling schemes for precious metals should be developed for recovering precious metals in urban mines to ensure stable supply of gold resources in Japan.
| Figure 4: Percentages of Different Supply Sources of Gold (total: 162 tons in 2019, Japan)5) |
Figure 5: Gold Reserves in Major Countries (total: 54 kilotons)7) |
|---|---|
 |
 |
To summarize what we have said above, gold is demanded principally for jewelry and investment, and there is also demand for gold in substantial amounts in industrial fields, especially in Japan. The high prices of precious metals will further encourage industries to use smaller amounts of such metals and proactively shift to alternative materials in order to reduce production costs. Nonetheless, there will probably not be any dramatic fall in demand for precious metals since new products using the materials characteristics of precious metals are being manufactured. Regarding the supply side, new domestic production of precious metals alone is not able to meet the entire domestic demand. Imports from foreign countries are unavoidable, but a far greater amount of gold has been accumulated in urban mines in Japan. One of the issues to be tackled is the development of such urban mines to ensure the stable supply of gold in domestic markets.
For that purpose, there needs to be recycling processes for precious metals including gold that are sophisticated and optimized to recover and refine such metals in an economical manner.
2. Flow of precious metals recycling
Precious metals wastes to be recycled are in solid or liquid forms. For example, production scrap (from electronic substrate and ICs, etc.), automobile exhaust gas catalysts, and dental materials are in solid form. Plating waste, etching waste, and catalytic waste are in liquid form.
Components and jigs such as adhesion preventing plates and metal masks which have been used in manufacturing processes and to which precious metal adheres are delivered as solid form wastes. Such components and jigs can be reused after the precious metals adhering to them are separated. The precious metals are separated using chemicals and appropriately cleaned before the components and jigs are returned to the relevant customers.
Figure 6 illustrates a typical flow of precious metals recycling.
Figure 6: Flow of Precious Metals Recycling4)
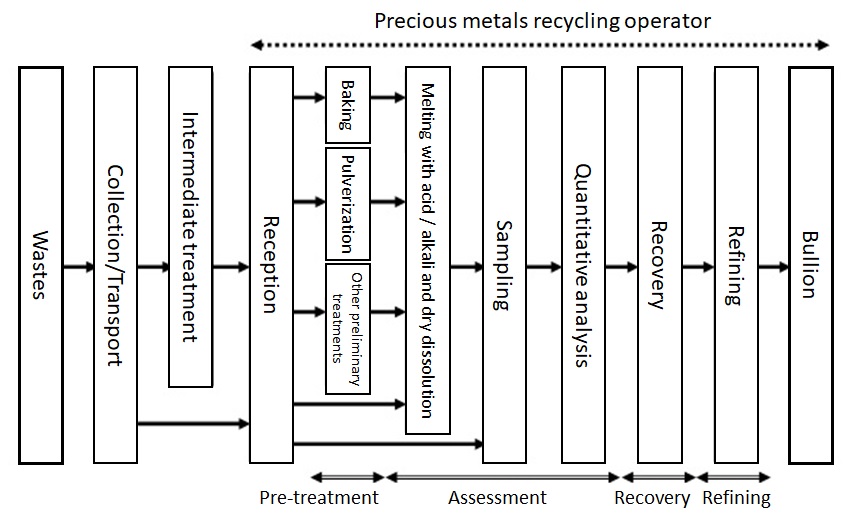 |
First, wastes that have been produced are collected and transported from customers (and subjected to intermediate treatment as required) and are transported to a metal recycling operator. It should be noted that wastes must be packed appropriately and the applicable laws and regulations must be observed. For example, the weight of hygroscopic wastes may increase from absorbing moisture during transportation so such wastes should be sealed in a waterproof bag to prevent them from absorbing moisture. The Fire Service Act is applicable to wastes that are designated as hazardous materials. The amount of such wastes that can be transported at one time needs to be limited to the specified amount.
Collected wastes are weighed or counted to make sure that there are no differences from the weight or amount held for customers. They are also subjected to X-ray inspection and pH tests to check that they have no unexpected components when they are collated with the relevant information provided by customers. This work is conducted to safely complete the downstream operations in the assessing, recovering and refining processes.
The wastes accepted in these operations are then subjected to processes for assessing the amounts of precious metals contained. The most important factors for correct assessment of precious metals are “homogenization” and “high-accuracy assay” of precious metals.
The following is a general description of the flow of assessment of wastes. First, wastes are homogenized. For this purpose, melting (liquefaction) with acid or alkali, pulverization, and/or fusion by dissolution can be adopted. Wastes must be subjected to prior treatments if they contain substances that may inhibit homogenization. For example, if wastes contain cutting oil on chips and resin in printed circuit boards that will negatively affect their melting and pulverization, such organic substances should be eliminated by baking. Other treatments are performed for facilitating the homogenization: removal of base metals by wet processing and separation of precious metal particles in dispersion liquid by coagulation treatment. Homogenization is followed by appropriate sampling. Samples are used for assaying precious metals. Assaying precious metals must be highly accurate. Chemical gravimetric analysis combined with instrumental analysis is conducted to implement highly accurate assaying. Chemical gravimetric analysis is an assaying method of separating precious metals from the sample in a chemical reaction process and measuring their weights directly. Cupellation, a gold purity assaying method, is also a chemical gravimetric analysis. On the other hand, instrumental analysis is a method of determining analytical results by comparing the output values for a reference material for which concentration is known and an analysis sample to the output values of luminescence intensity and absorbance obtained with an analytical instrument. TANAKA Kikinzoku Kogyo uses relevant assaying instruments according to the analysis target: inductivity coupled plasma optical emission spectrometer (ICP-OES), X-ray fluorescence instrument, atomic absorption photometer and glow-discharge mass spectrometer.
Analytical results obtained in these assays are converted to the required matrix to determine the amounts of precious metals contained in the wastes. The customer is notified of these amounts. After the customer’s approval of the amounts, the wastes are subjected to recovery and refining processes.In the recovery and refining processes, solutions, powders and ingots are subjected to wet and dry processes to refine precious metals up to purities that are suitable for products. These processes vary depending on the form of the waste and will be described in the following section.
Precious metal bullions produced in the recovery and refining processes are returned to customers at their request. Three possibilities are available: return of the as-recovered metal, return of an amount of money equivalent to the recovered metal, or return of products made from the recovered metal.
3. Recovery and refining processes
Wet treatments and dry treatments are available for recovery and refining of precious metals. The former treatments are described below since they are applied generally.
Wet treatments in the recovery and refining processes are broadly divided into three stages: “liquefaction,” “recovery” and “refining.”
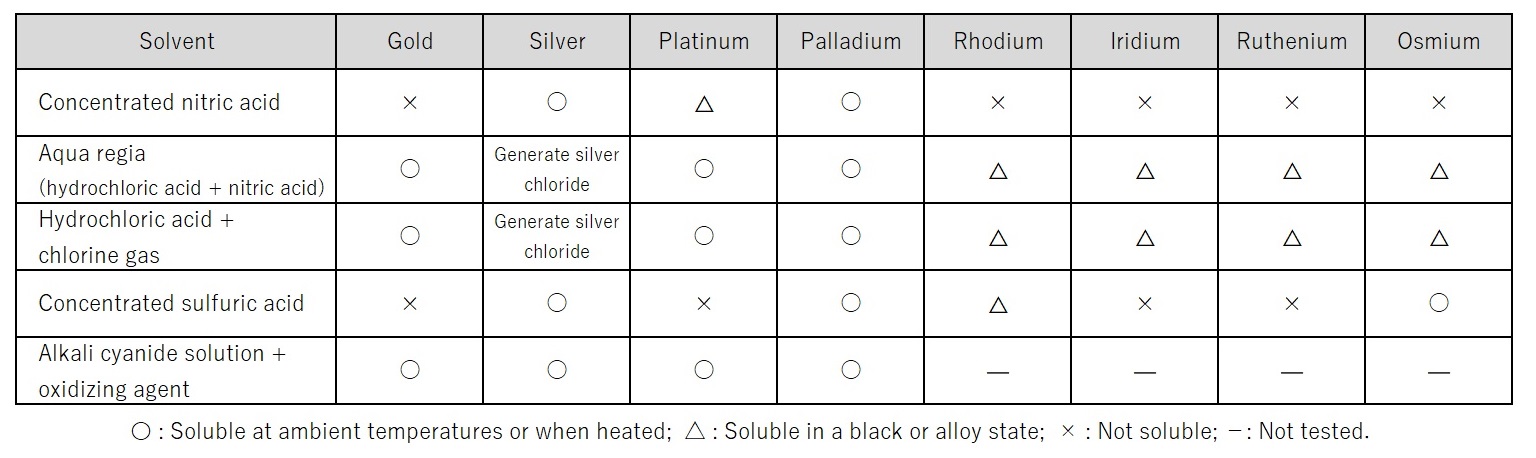
First stage: Liquefaction partly overlaps with the liquefying treatment in the assessment process for wastes. In the recovery and refining processes, liquefaction is the process of dissolving precious metals contained in powder or ingots in a chemical agent to ionize them. A suitable chemical agent is chosen from nitric acid, aqua regia (hydrochloric acid + nitric acid) and cyanogen-containing liquid according to the types/composition of the precious metals (Table 1). A strong oxidizing agent is used to dissolve precious metals that are chemically stable materials. As needed, other metals are added to the waste material to adjust its composition so that it becomes more dissolvable in a chemical agent.
Table 1: Liquefaction of Precious Metals11)
 |
Second stage: Recovery is the process for rough separation of precious metals from impurities, as a preliminary treatment for refining. Specifically, several separation techniques are applied such as adsorption, cementation, precipitation, and electrolytic extraction. A suitable technique should be chosen according to the concentrations and types of precious metals in the liquid of dissolved wastes (Table 2).
Table 2: Wet Processes for Recovery and Refining of Precious Metals4)
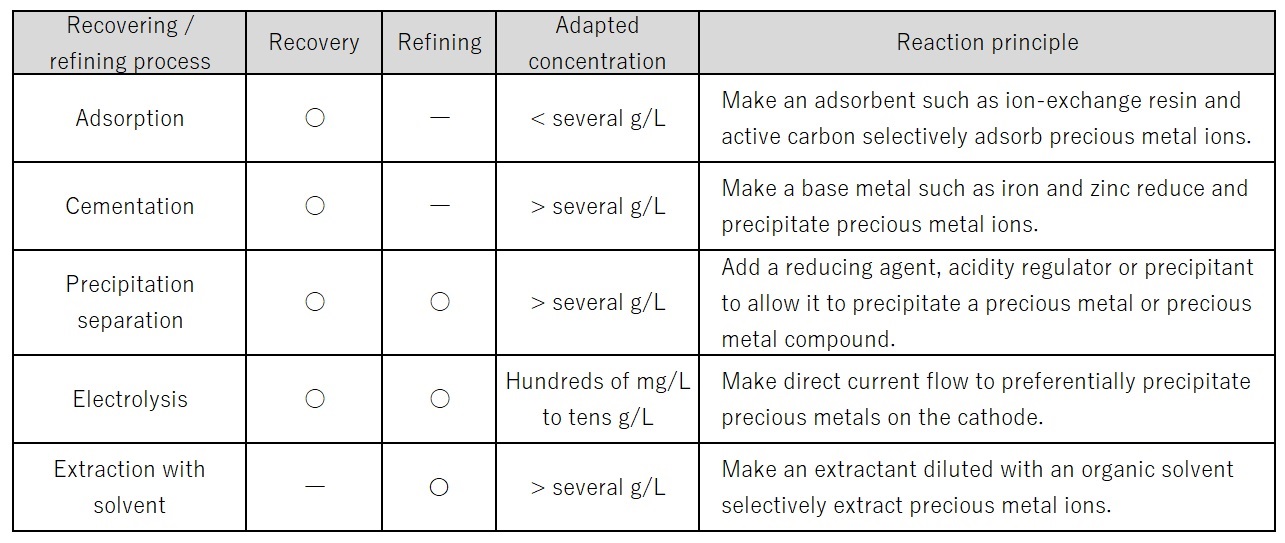 |
Third stage: Refining is the process for purifying the precious metals roughly separated in the recovery process to a level that is suitable as raw material for products. Examples of separation techniques are precipitation separation, electrolytic refining, and extraction with a solvent. To separate and eliminate impurities in the material, their types and concentrations should be correctly identified and the impacts of control factors such as amount of chemical agent, reaction temperature and duration on the purities of precious metals should be understood.
The recovery and refining processes for precious metals are required to reduce their loss to the minimum. Designing these processes should be based on a combination of several separation techniques. For example, liquids of dissolved wastes with a high concentration of precious metals are subjected to reduction manipulation for precipitation to separate them from impurities. If the total amount of precious metals is reduced to enhance the yielding percentage, as impurities are also reduced and enter the resulting material, the purities of resulting precious metals are lowered. For this reason, the input of the reducing agent should be controlled to leave certain traces of precious metals in the liquid. The remaining precious metals are recovered by adsorption manipulation.
The wet treatments based on these techniques have several issues to be resolved. One of the major issues involved is the treatment of the effluent after recovery of precious metals. The possibility of residual oxidizing agent can be a problem during draining since a strong oxidizing agent is used for dissolving precious metals.
One of the problematic chemical agents is nitric acid. This agent is used to prepare aqua regia for dissolving silver and gold. It is not totally dissolved and remains in the form of nitric acid ion in the effluent after recovery of precious metals. The Water Pollution Prevention Act establishes effluent standards.
The nitric acid ions emitted by the precious metal manufacturing/recycling industry may not exceed a nitrate-nitrogen concentration of 2,800mg/L provisionally for July 2019 to June 2022. In the future, a stricter standard (100mg/L) will be applied to all industries. Industrial processes that emit no nitric acid should be adopted. 12)
Precious metals recycling process (example)
We present below an example of a precious metals recycling process and related issues.
1. Clad materials
Clad materials are materials composed of at least two dissimilar metal sheets.
These metal sheets are firmly bonded by rolling and sintering, with diffused phases of metals formed. This bonding structure is more resistant to peeling forces than plating.
In general, precious metals are used on terminals of electronic parts because of their corrosion resistance. To economize on use of such expensive metals, a solution has been invented: precious metals are used only on parts that require such metals and alternative metals such as copper and tin are substituted on remaining parts. Clad materials as shown in Figure 7 have been manufactured as materials for electronic parts. Such clad materials are partially composed of precious metals. The majority of the clad material shown in Figure 7 is composed of a base metal such as copper or tin. The portion of precious metal only accounts for a small percentage. If such waste materials are liquefied in the previously mentioned assessing process using chemical agents, a large amount of liquid with a low concentration of precious metals would be produced. Precious metals could be recovered from such liquid with a low concentration of precious metals by adsorption or electrolytic treatment. However, it would take a long time to process such a large amount of liquid and would be less economical and less environment-friendly. Thus, a preliminary separation of base metals should be completed before the chemical dissolution process.
Figure 7: Structure of Clad Material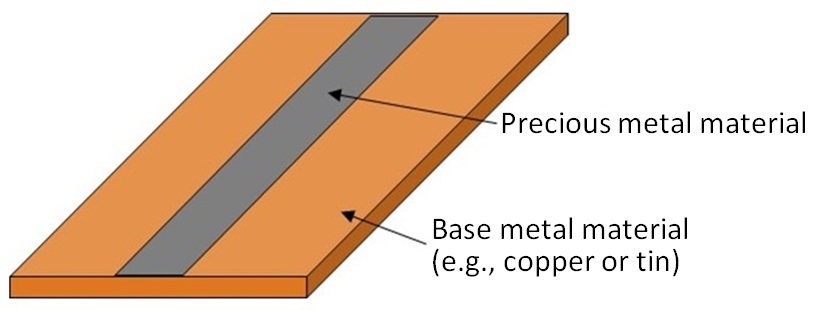 |
A solution to this issue would be to utilize ionization tendency. Table 3 shows the standard electrode potentials of major metals. Higher potential corresponds to nobler property or lower susceptibility to corrosion. Precious metals (also known as “noble metals”) are nobler than copper and tin. When two heterophase metals are in contact with each other, bimetallic corrosion (galvanic corrosion) may occur. This is the phenomenon that when two heterophase metals are contact with each other in the presence of an electrolytic solution, the baser will selectively corrode. The same phenomenon can occur in clad material composed of bonded heterophase metal sheets. This phenomenon and the noble property are utilized to eliminate base metals.
Table 3: Standard Electrode Potentials of Major Metals13)
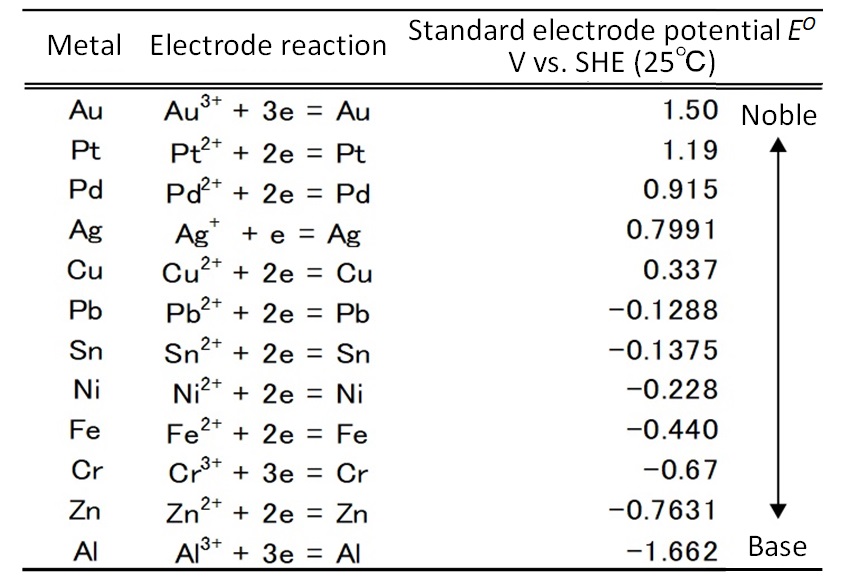 |
2. MEA
MEA (membrane electrode assembly) is a component of fuel cells. Figure 8 illustrates a typical structure of MEA14): lamination of platinum-supporting carbon particles on proton exchange membrane. Hydrogen gas is supplied to the anode side of MEA to obtain electrons by separating them from hydrogen ions (protons) on platinum as catalyst. Hydrogen ions move around in the proton exchange membrane, react with hydrogen at the cathode side and turn into water. A fuel cell discharges nothing but water and is recently attracting attention as a clean power generation system. The platinum used in MEA should be recycled as a matter of course in the precious metal recycling scheme. An adequate technique for dissolving the platinum should be developed.
Figure 8: Structure of MEA
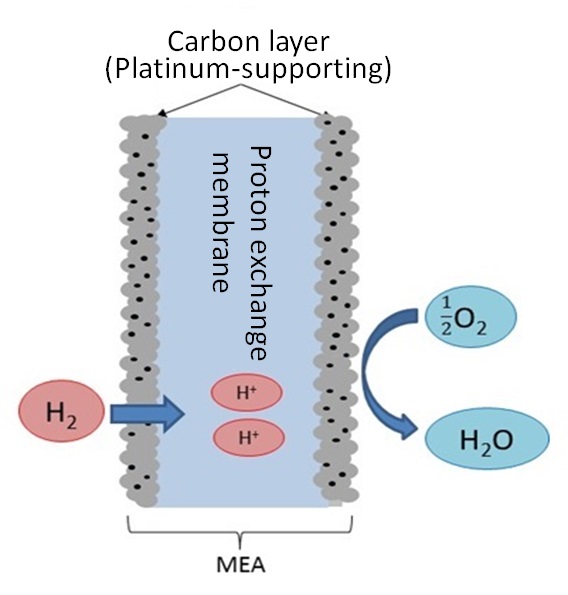 |
Conclusion
This report has described precious metals recycling from the perspectives of demand and supply of precious metals, flow of precious metals recycling and recovery and refining processes. Newly produced domestic gold does not meet the whole domestic demand for this precious metal in Japan. This makes it essential to recover and refine precious metals from urban mines. Development of recycling processes that are less costly for recovery and refining of precious metals and also environment-friendly is required more than ever.
Recently, there is also a concern that the tendency to use less amounts of precious metals and the complicated material composition of precious metal containing wastes might raise processing costs in the existing recycling processes. An issue to be resolved in the future will be enhancement of pre-treatment techniques for separating and removing pollutants and impurities in order to construct a more efficient recycling system.
Precious metals are highly useful to people and will remain an essential part of resources for society while being used in various fields and settings. Being engaged in the precious metals recycling business, we hope to continue daily research activities in order to find recycling processes that excel in safety, quality, environmental impact and economical aspects when handling different forms of waste materials.
References
1) Michinori Ooki, Toshiaki Oosawa, Motoharu Tanaka and Hideaki Chihara: Dictionary of Chemistry, 1st edition, Tokyo Kagaku Dojin.
2) Susumu Shimizu and Yukihiro Muragishi: Illustrated ABCs of Basics of Precious Metal Utilization Technologies, 1st edition, Nikkan Kogyo Shimbun (2011).
3) Thomson Reuters: GFMS Gold Survey 2019, digest version in Japanese, p.8, TANAKA Kikinzoku Kogyo (2018).
4) Kazuaki Tsuchiya: Journal of the Society of Inorganic Materials, Japan, Vol. 27, p.25-30 (2020).
5) Agency for Natural Resources and Energy, Ministry of Economy, Trade and Industry: Statistical Survey of Distribution of Precious Metals (2012/2019).
6) Akihiro Yoshimura and Yasunari Matsuno: Journal of The Japan Institute of Metals and Materials, Vol. 78, No. 8, p.303-309 (2014).
7) U.S. Department of the Interior, “MINERAL COMMODITY SUMMARIES 2019”, p.71 (2019)
8) Japan Oil, Gas and Metals National Corporation, IAA: Material Flow of Mineral Resources 2018 – Gold (Au).
9) K. Okada: Chishitsu News, No.601, p.16-27 (2004).
10) National Institute for Materials Science, IAA, https://www.nims.go.jp/news/press/2008/01/p200801110.html (2019.07.04)
11) J. Shibata, A. Okuda: Shigen-to-Sozai, Vol.118, p.1-8 (2002).
12) Takanori Kimura: Proceedings of the 8th session of the Precious Metal Symposium, p.37-45 (2021).
13) The Chemical Society of Japan, compiler: Handbook of Chemistry – Basics, 3rd revised edition, p.II-474-II-476, Maruzen Co. Ltd. (1984).
14) Yoshiyuki Hashimasa, Yoshiyuki Matsuda, Daichi Imamura, Motoaki Akai, and Masafumi Sasaki: collection of papers of The Japan Society of Mechanical Engineers, Part B, Vol. 77, No. 773, p.147-159 (2011).
![]()









