CLOSE
About Elements
TANAKA is a leading company in the field of precious metals.
Advanced materials and solutions that support societal progress, the development stories behind them, the voices of engineers, and our management philosophy and vision—
Elements is an online media platform that shares insights that lead to a better society and a more prosperous future for the planet under the slogan “Mastering Precious Metals.”

Electric vehicle batteries could get big boost with new polymer coating
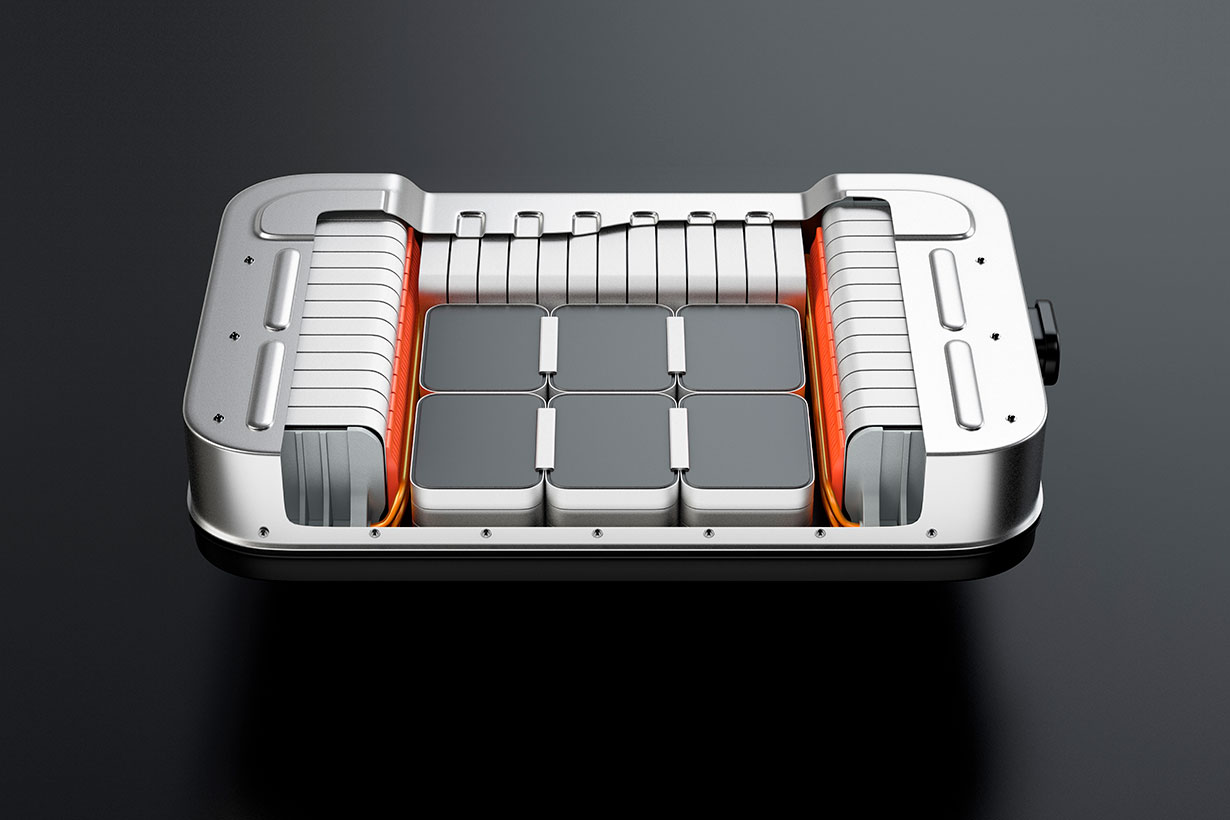
Scientists at Lawrence Berkeley National Laboratory (Berkeley Lab) have developed a conductive polymer coating – called HOS-PFM – that could enable longer lasting, more powerful lithium-ion batteries for electric vehicles.
“The advance opens up a new approach to developing EV batteries that are more affordable and easy to manufacture,” said Gao Liu, a senior scientist in Berkeley Lab’s Energy Technologies Area.
The HOS-PFM coating conducts both electrons and ions at the same time. This ensures battery stability and high charge/discharge rates while enhancing battery life. The coating also shows promise as a battery adhesive that could extend the lifetime of a lithium-ion battery from an average of 10 years to about 15 years, Liu added.
To demonstrate HOS-PFM’s superior conductive and adhesive properties, Liu and his team coated aluminum and silicon electrodes with HOS-PFM, and tested their performance in a lithium-ion battery setup.
The HOS-PFM conductive binder is made of a nontoxic polymer that transforms at the atomic level in response to heat. Before heating: At room temperature (20 degrees Celsius), alkyl end-chains (black squiggly lines) on the PFM polymer chain limit the movement of lithium ions (red circles). After heating: When heated to about 450 degrees Celsius (842 degrees Fahrenheit), the alkyl end-chains melt away, creating vacant “sticky” sites (blue wavy lines) that “grab” onto silicon or aluminum materials at the atomic level. PFM’s polymer chains then self-assemble into spaghetti-like strands called “hierarchically ordered structures” or HOS. Like an atomic expressway, the HOS-PFM strands allow lithium ions to hitch a ride with electrons (blue circles). These lithium ions and electrons move in synchronicity along the aligned conductive polymer chains. See GIF here (Credit: Jenny Nuss/Berkeley Lab)
Silicon and aluminum are promising electrode materials for lithium-ion batteries because of their potentially high energy storage capacity and lightweight profiles. But these cheap and abundant materials quickly wear down after multiple charge/discharge cycles.
During experiments at the Advanced Light Source and the Molecular Foundry, the researchers demonstrated that the HOS-PFM coating significantly prevents silicon- and aluminum-based electrodes from degrading during battery cycling while delivering high battery capacity over 300 cycles, a performance rate that’s on par with today’s state-of-the-art electrodes.
The results are impressive, Liu said, because silicon-based lithium-ion cells typically last for a limited number of charge/discharge cycles and calendar life. The researchers recently described these findings in the journal Nature Energy.
The HOS-PFM coating could allow the use of electrodes containing as much as 80% silicon. Such high silicon content could increase the energy density of lithium-ion batteries by at least 30%, Liu said. And because silicon is cheaper than graphite, the standard material for electrodes today, cheaper batteries could significantly increase the availability of entry-level electric vehicles, he added.
The team next plans to work with companies to scale up HOS-PFM for mass manufacturing.
Research Report:Formation of hierarchically ordered structures in conductive polymers to enhance the performances of lithium-ion batteries
This article was from SpaceDaily.com and was legally licensed through the Industry Dive Content Marketplace. Please direct all licensing questions to legal@industrydive.com.