Medical Uses
Diagnostics
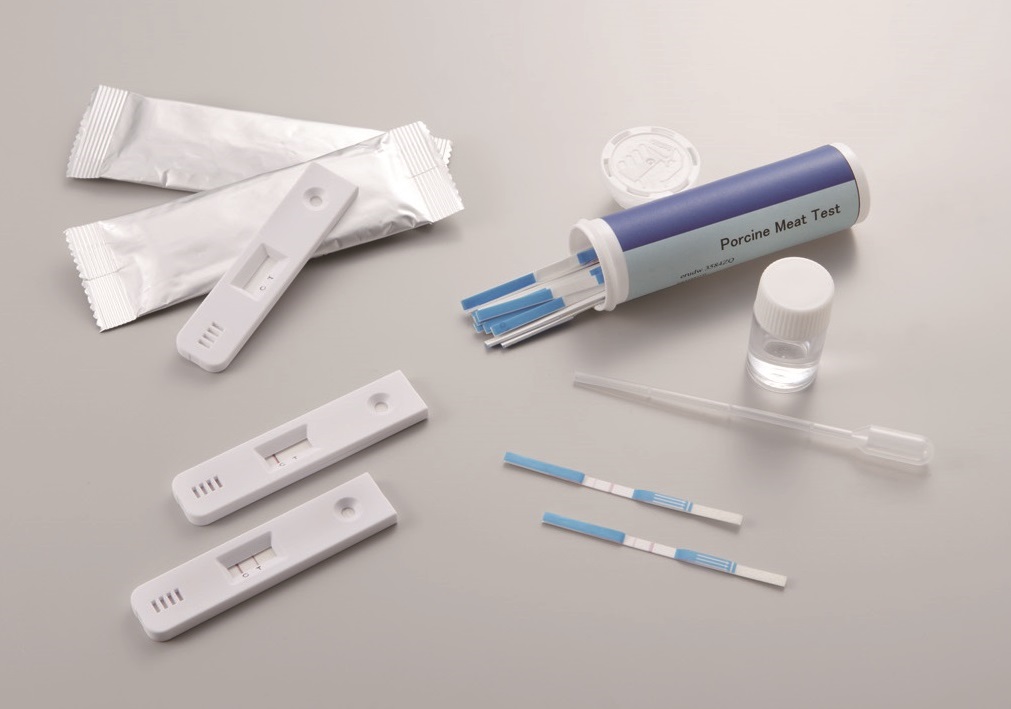
We manufacture in-vitro diagnostic and other test kits using precious metal colloids on a consignment basis.
In addition to the gold colloid manufacturing technologies it has built up over a number of years, TANAKA has at its disposal every possible technology relating to the highest performance immunochromatographic diagnostic test kits. These technologies include those which allow proteins to bind to gold colloids, prevent non-specific absorption of proteins by gold colloids, and promote antigen-antibody reactions.
TANAKA not only supplies raw materials for in-vitro diagnostic test kits, but also manufactures them on an original equipment manufacturer (OEM) basis under a reliable production system managed in accordance with the ISO 13485 system.
Consignment details
- Conjugated gold colloid solution
- Development and manufacture of inspection test strips (immunochromatographic method*)
- OEM manufacture



Registration and certification
- Registered for the manufacturing of in-vitro diagnostics
- ISO 13485 certified

*Detection principle using immunochromatographic method
If antigens (such as virus antigens) are present in a specimen, gold colloids bind to the gold colloid-labeled antibodies to form composite bodies. The
composite bodies then pass through a membrane as a result of the capillary action and bind to the capture antibodies that are immobilized in the detection area. These then appear as a red band.
This principle is used to determine
whether antigens are present or not.
Test Strip Illustration
![[Test Strip Illustration] (Sample flow) From left: Specimen, Gold colloid, Gold colloid-labeled antibody, Capture antibody](https://prod-cms-cache-bucket.s3-ap-northeast-1.amazonaws.com/wp-content/uploads/sites/images/ex/en/products/images/f03/img_block02_01.jpg)
Reel-to-reel system

Automatic packaging system


Automatic wrapping system

Product inspection

